Principal Investigator: Gerald Tramontano, Ph.D. and the clinical research team at NCBI and the NCI Clinical Research Foundation are conducting a research study evaluating the efficacy of enhancing standard behavioral therapy with non-invasive neuromodulation (NM) in treating anxiety.
Study Description: This is a non-invasive neuromodulation treatment clinical research study in children and adults. Similar devices, previously called CES (cranial electrical stimulators) have been approved by the US FDA for the treatment of anxiety, depression and insomnia in adults in the 1970s. Since that time, medical device technology has vastly improved. Leveraging those technological advancements, this study is collecting clinical and safety data for an FDA trial showing that this CES device is equally safe and as effective or more effect and to ultimately seek an FDA approval for the treatment of anxiety in pediatrics.
The study will be conducted in 3 stages:
Stage 1 and 2 are the randomized double blinded stages. Each stage lasts for 4 weeks, requires 18 visits to the clinic for treatment sessions which is a combination of behavioral therapy and neuromodulation. There is a 50% chance of being in the placebo/sham group – during these stages. The patient and clinician will not know if the patient is on the placebo or treatment group. You are guaranteed to receive the treatment device being studied in stage 1 or 2.
Stage 3 (Open-label, optional) – will last 8 weeks and the patient can take one of the neuromodulation devices home and can use the treatment daily.
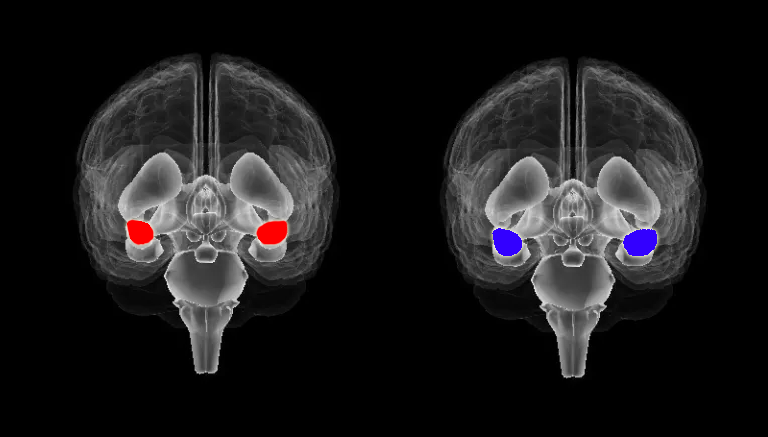
All research participants will receive ANS testing, cortisol and neurotransmitter assessment, resting state functional brain mapping using electrical neuroimaging and undergo comprehensive neuropsychological testing as part of this clinical study. There is also an fMRI sub-study.
Study Participants: This study is screening patients, age 5 and older, with anxiety-related disorders such as separation or social-based anxiety, generalized anxiety, OCD, PTSD and patients with other heightened sympathetic ANS over-arousal symptoms and disorders.
Watch video: Overview of the AMG 05 Anxiety Disorders Clinical Trial
For more information, you may (973) 601-0100 Option 6 or email research@neuroci.com.